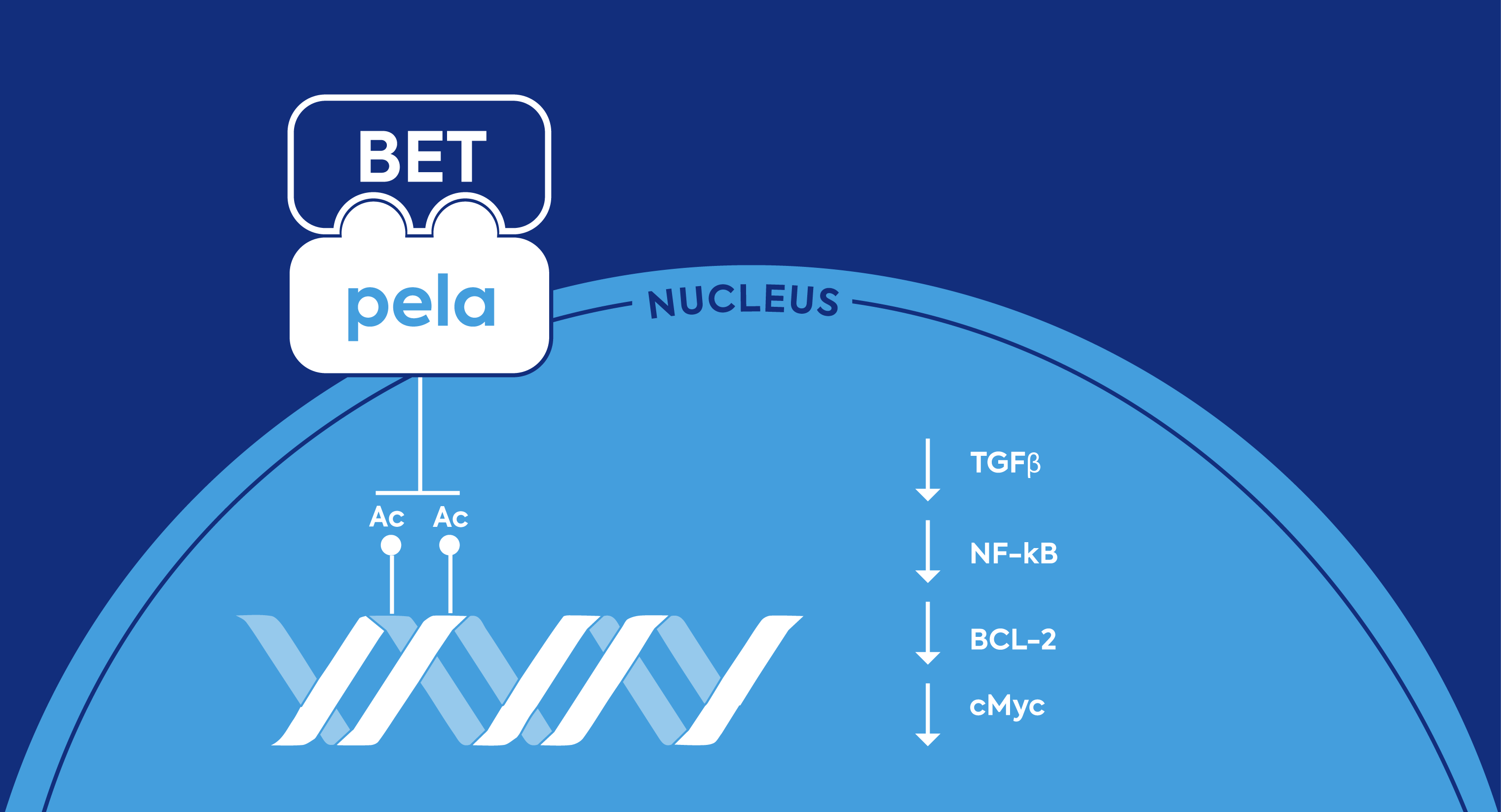
Pelabresib is an investigational selective small-molecule therapy aimed at promoting anti-tumor activity. It is designed to inhibit bromodomain and extra-terminal domain (BET) proteins, which may downregulate genes implicated in blood cancers.
Pelabresib Inhibits BET Proteins
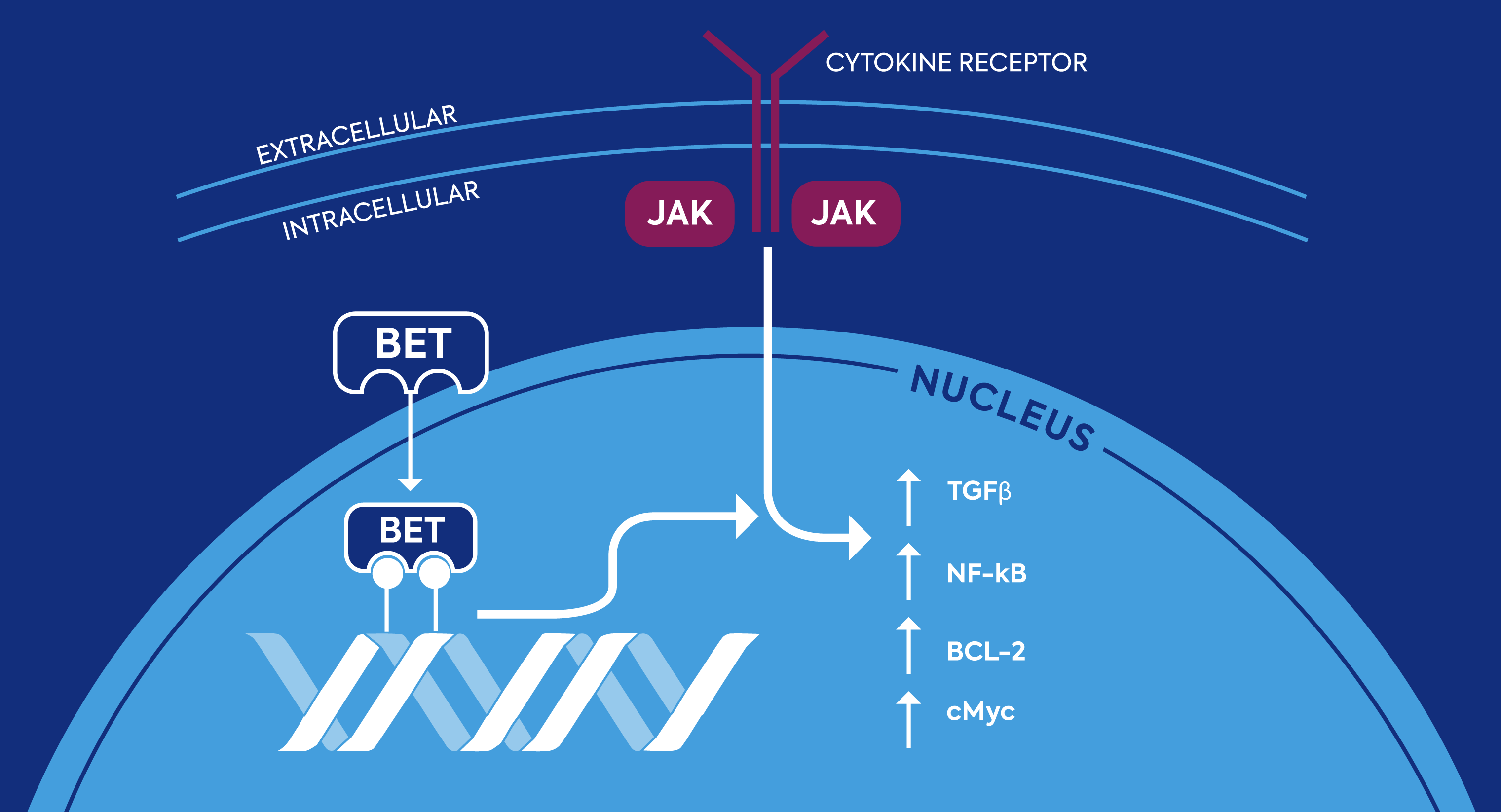
The BET family of proteins, along with the JAK/STAT pathway, have been implicated in the development and progression of myelofibrosis. Both pathways may increase levels of pro-inflammatory cytokines and stimulate production of abnormal blood cell precursors called megakaryocytes. The BET family of proteins is therefore emerging as a promising therapeutic target whose modulation may alter the underlying cause of disease in myelofibrosis, with possible synergistic results alongside JAK inhibition.
The JAK–STAT Pathway and BET Proteins Have Been Implicated in the Development of Myelofibrosis
Pelabresib is currently being investigated in combination with the JAK inhibitor ruxolitinib compared with placebo plus ruxolitinib in JAK inhibitor-naïve patients with myelofibrosis (those who have not been previously treated with a JAK inhibitor) in the Phase 3 MANIFEST-2 study and in Arm 3 of the Phase 2 MANIFEST study. A full list of the MANIFEST study arms can be found below.
Beyond myelofibrosis, pelabresib has demonstrated potential clinical benefit in early-stage trials in other myeloid diseases. We look forward to investigating pelabresib further in lower-risk myelodysplastic syndromes (MDS) and essential thrombocytopenia (ET).
The development of pelabresib was funded in part by The Leukemia and Lymphoma Society®.

