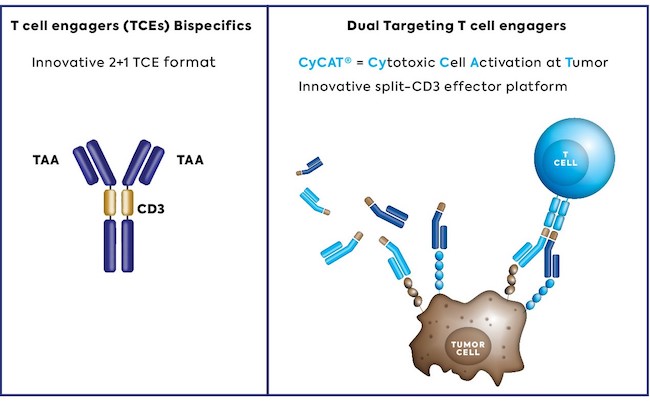
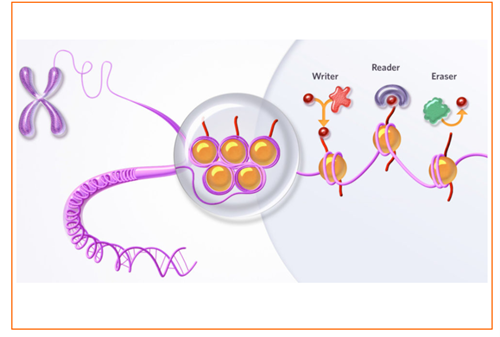
Although there are several technologies currently available for the discovery of therapeutic antibodies, they can make this process difficult in two ways. Many antibody programs fail in later development due to inadequate biophysical properties – and if an antibody cannot be produced on a larger scale, or if other properties adversely affect its handling, the chances of successful commercialization decrease.
In addition, earlier technology generations fail to deliver antibodies against all possible targets (epitopes) of disease-relevant target molecules. A lack of structural diversity in the generation of antibodies means that not all possibilities to bind to a promising target molecule are exhausted and its role in the course of the disease is underestimated.
Ylanthia is a technology that has been specifically designed to overcome these hurdles, thereby going one step further than all current antibody technologies.